Researchers Reveal the Secrete of Nonrandom DNA Seg-regation in Human Cells
Sepsis is a life-threatening organ dysfunction driven by a dysregulated host response, which affects approximately 50 million individuals and causes over 11 million fatalities worldwide annually. Sepsis-induced cardiomyopathy (SICM) is a common and important cause of death in patients with sepsis. Patients usually display the reduced ejection fraction, abnormal ventricular dilatation, poor response to fluid therapy and cardiovascular active agents. Notably, patients with SICM have a transient and significant improvement in cardiac function,suggesting that cardiac homeostasis can be restored after the septic stress. However, the underlying mechanisms facilitating the heart rehabilitation during sepsis have not been fully elucidated.
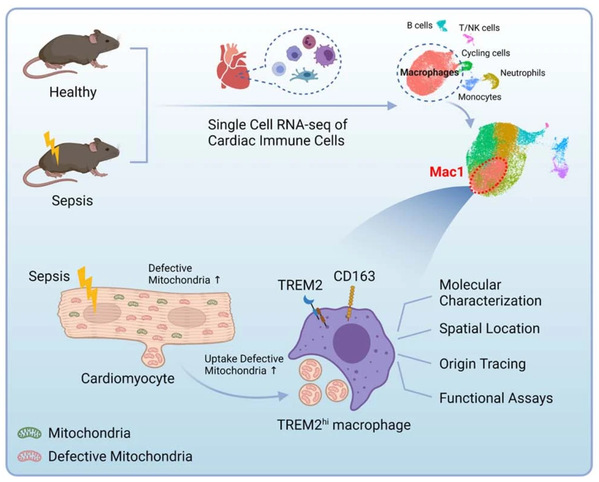
On January 12, 2023, Prof. FANG Xiangming’s team from the First Affiliated Hospital, Zhejiang University School of Medicine published an article entitled “TREM2hi resident macrophages protect the septic heart by maintaining cardiomyocyte homeostasis” in the journal Nature Metabolism. This study shows that bacterial sepsis induces marked but transient changes in the immune compartment of the mouse heart, including the disappearance of a type of tissue-resident macrophages that protects the heart by scavenging defective mitochondria released by cardiomyocytes.
Which immune cell subset is critical for SICM?
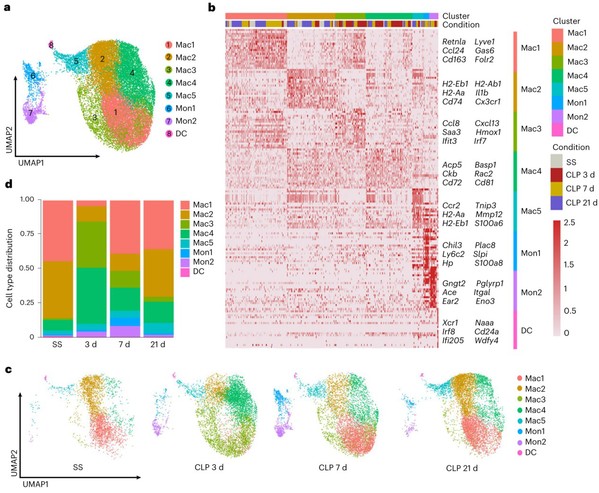
Cardiac immune cells are key regulator of heart microenvironment and closely related to cardiac homeostasis and diseases. So, two questions are coming up: how do cardiac immune cells change in SCIM? and which is the SCIM-associated essential cell subset? To address these questions, using flow cytometry and single cell RNA-sequencing (scRNA-seq), the team analyzed 29537 cardiac immune cells from hearts of 14 wild-type (WT) mice at steady state (SS), and 3, 7 and 21 days post-CLP, respectively. They observed that macrophages were the most abundant immune cells in both WT and septic hearts, and display a dynamic change following sepsis. Intriguingly, one macrophage subset (Mac1) reduced first and recovered later on following sepsis. Mac1 subset is characterized by the expression of Trem2, Retnla, Lyve1, Cd163 and Folr2, and resembled the transcriptomic signatures of cardiac tissue-resident macrophages. The finding that Mac1 subset associating with the recovery of cardiac function in sepsis prompts us to explore whether Mac1 is the Angels or the Demons in SICM.
Fig. 1: A distinct cardiac macrophage subset(Mac1) is associated with the recovery of SICM.
Which gene is the key regulator of SICM-associated macrophage (Mac1)?
The RNA-seq data analysis revealed that genes upregulated in Mac1 were related with phagocytosis and endocytosis in biological terms, including the triggering receptor expressed on myeloid cells 2 (Trem2). Trem2, which is mainly expressed on the surface of macrophages, regulates the function of macrophages, and involves in pathogen phagocytosis, anti-inflammatory mediators’ production, lipid and energy metabolism10-13. Importantly, the team observed Trem2 is highly expressed in Mac1 cells and essential for Mac1 remodeling during SICM. The proportion of Mac1 cells both significantly decreases in WT and Trem2-/- mice 3 days post-CLP. 7 days post CLP, Mac1 cells recovers to normal level in WT mice, but Trem2-/- mice could not restore the proportion of Mac1 cells, suggesting a key role of Trem2 in Mac1 cells. The study further showed that Trem2 deficiency impairs the proliferative capability of Mac1 cells.
What’s the function of TREM2hi Mac1?
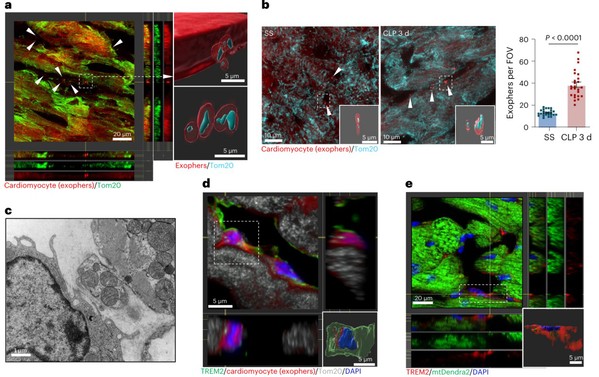
The fate mapping data strongly support that Mac1 cells are self-renewing resident macrophages. Nicola´s-A´ vila et al. has implied that cardiac resident macrophages could engulf a type of extracellular particles (known as exophers) which contain defective mitochondria, and preserve mitochondrial function in the heart. The research team wonder whether TREM2hi Mac1 could protect heart function in sepsis.To address it, they constructed αMHCCre:Rosa26TdTom mouse line to track cardiomyocyte-derived exophers, as well as αMHCCre:mtD2Flox/Flox mouse line and AAV-Tnnt2-mtKeima to track cardiomyocyte-derived mitochondria. 3D reconstruction and video showed that TREM2hi Mac1 scavenged exophers and defective mitochondria released from cardiomyocytes. Furthermore, Trem2 deficiency leads to the failure of removal these defective mitochondria and consequently accelerates heart injury.
Fig. 2: TREM2 promotes the uptake of cardiomyocyte-derived mitochondria
by Mac1 in septic heart.
How to understand the value of SCIM-associated macrophage subset?
The study illustrate immune cells of heart under steady state, sepsis progression and recovery using single cell RNA-seq and reveal a subset of macrophage are positively related to cardiac function. It then shows that the high expression of TREM2 is the key feature of these SCIM-associated macrophages and clarify their roles in septic heart. In addition, the transplantation of Mac1 subset can ameliorate the cardiac dysfunction.
Investigating the SCIM-associated macrophage subset can not only help to better understand the pathogenesis of septic heart, but also accelerate the transformation of macrophage immunotherapy. Identification of the specific markers and function of disease-associated macrophage will provide the direction of cell therapy, including designing the specific cell-killer CAR-T or transplantation of functional cells. The methods of manipulating macrophage subsets both of tissue resident macrophages or monocyte-derived macrophages in vivo and in vitro reveals the origins and plasticity of macrophage. iPSCs derived specific macrophage subsets could play important roles in popularizing macrophage immunotherapy in sepsis. Medicines targeting macrophage activity to destroy/enhance their function are relied on detailed characterization of macrophage subsets. Although there is a long way to go, it’s worthy to overcome these impediments to realize it in sepsis precise treatment.
In summary, Prof. FANG’s team find TREM2hi Mac1 removes exophers released from myocardium to maintain homeostasis of septic heart and improves the outcome of SICM murine model. The study lightens the future way in application of cellular targeted therapy including SICM-associated Mac1 subpopulations. Although there is a long way to go, it’s worthy to overcome these impediments to realize it in sepsis precise treatment.
More information: Dr. ZHANG Kai, Ph.D. candidates WANG Yang, CHEN Shiyu and MAO Jiali are the co-first authors of this article. Prof. FANG Xiangming, CHEN Qixing and LI Xuekun are the co-corresponding authors of this article.
Source: The First Affiliated Hospital, Zhejiang University School of Medicine