Researchers Reveal the Secrete of Nonrandom DNA Seg-regation in Human Cells
Blocking cell-cycle checkpoints by pharmacologic or genetic disruption of checkpoint kinase activities can force cell-cycle progression even in the presence of damaged DNA, and represents a promising antitumor therapeutic strategy. Indeed, potent inhibitors for several cell-cycle checkpoint kinases including ATR, ATM, CHK1, CHK2, and WEE1 are clinically evaluated. Preclinical studies, however, reveal that the therapeutic efficacy of checkpoint kinase inhibition is context-sensitive and depends on a variety of factors, including the unique genetic makeup of individual patient. Specific biomarkers for predicting responses to WEE1 inhibitor remain to be identified.
On January 12, the research team led by LIU Ting at the Zhejiang University School of Medicine published a research article titled “SIRT1 deacetylates WEE1 and sensitizes cancer cells to WEE1 inhibition” in Nature Chemical Biology, indicating that SIRT1 expression level and abundance of WEE1 Lys177 acetylation in tumor cells can serve as useful biomarkers for predicting WEE1 inhibitor sensitivity or resistance.
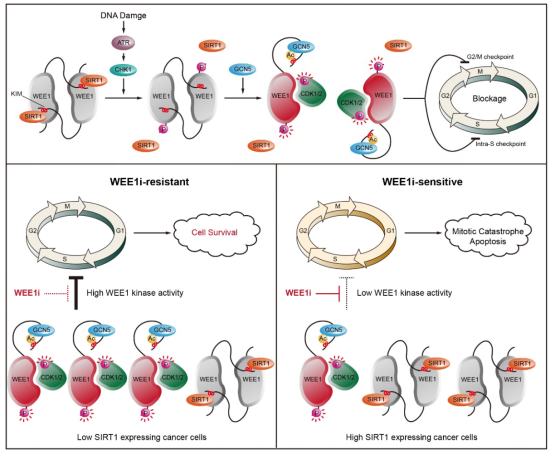
In their study, the researchers discovered a previously unidentified regulatory mechanism for the catalytic activity of WEE1 involved in the intra-S-phase and G2/M checkpoints. They identified an evolutionarily conserved kinase inhibitory module (KIM) within the N-terminal regulatory domain. In the non-activated state, WEE1 forms a dimer through the binding of KIM to the C-terminal kinase domain, thus maintaining low kinase activity. Upon DNA damage, CHK1 promotes WEE1 phosphorylation at Ser642, thereby priming GCN5-mediated acetylation at Lys177 and dissociating the inhibitory segment from the kinase domain and subsequent activation of WEE1 and cell-cycle checkpoints. Conversely, SIRT1 deficiency induces WEE1 hyperacetylation and activation, rendering cancer cells resistant to WEE1 inhibition.
Understanding the molecular details of WEE1 regulation may open the door to the biomarker-centered prediction of WEE1 resistance as well as the development of novel WEE1 inhibitors.