Excessive or repetitive fear play a significant role in the development of anxiety disorders, with the amygdala serving as the central locus for fear processing. Clinical research has demonstrated that individuals with bilateral amygdala damage are still capable of experiencing fear, indicating the amygdala may not be absolutely required for fear. To date, the neural mechanisms underlying fear that are independent of the amygdala are still poorly understood.
On February 12th, Prof. LI Xiao-Ming and his team from the Zhejiang University School of Medicine published an article entitled A molecularly defined amygdala-independent tetra-synaptic forebrain-to-hindbrain pathway for odor-driven innate fear and anxiety on Nature Neuroscience. The study revealed the significant role of the main olfactory bulb → dorsal peduncular cortex → lateral parabrachial nucleus → parasubthalamic nucleus pathway in fear and anxiety (Figure 1).
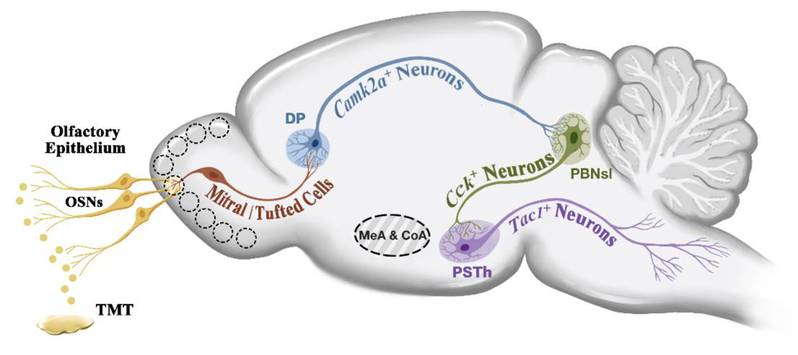
Figure 1 The schematic diagram of the main olfactory bulb → dorsal peduncular cortex → lateral parabrachial nucleus → parasubthalamic nucleus pathway
Olfaction serves as a common sensory modality that elicits innate fear in animals. Through the use of 2,4,5-trimethyl-3-thiazoline (TMT), a compound present in fox feces, which is a stimulus with fear-eliciting properties for rodents, the research team observed a notable decrease in aversive and freezing behaviors triggered by TMT in mice, accompanied by the apoptosis of neurons in the cortical amygdala and medial amygdala. However, this kind of apoptosis did not have a significant impact on TMT-induced escape behavior. Therefore the team focused on finding the specific brain region that mediate the olfaction-evoked escape behavior.
In the subsequent experiments, Dr. WANG Hao, the first author of the study, characterized neuronal activity as reflected in Fos expression in response to TMT. He observed a notable elevation in Fos expression in the dorsal peduncular cortex (DP), which receives distinct inputs from the main olfactory bulb (MOB). In addition, the MOB-DP neural circuit exhibits markedly heightened activity following TMT stimulation (Figure 2).
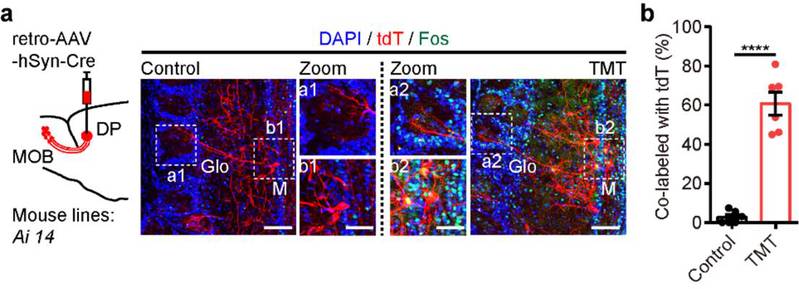
Figure 2The MOB-DP neural circuit is involved in TMT-induced innate fear.
The role of DP in olfaction-evoked innate fear was investigated by inhibiting DP neurons in mice using an apoptosis virus, which resulted in the absence of obvious escape behavior in response to TMT stimulation and a significant reduction in aversive and freezing behaviors. Conversely, activating DP neurons using optogenetics induced escape behavior in mice, along with observable fear-like reactions such as dilated pupils and decreased heart rate, explained Dr. WANG Hao.
Concurrently, the team integrated optogenetic inhibition of DP neuron function with localized amygdala damage in mice. They observed that the combination of localized amygdala damage and DP inhibition resulted in a significant reduction of escape behavior induced by TMT in mice, as well as a further decrease in aversive and freezing behaviors. Notably, the mitral/tufted cells projecting to DP and the cortical amygdala are two distinct groups of neurons. The aforementioned functional and structural observations suggest that DP is capable of autonomously mediating olfaction-evoked innate fear bypasses the amygdala, stated Dr. WANG Qinng, co-first author of the study. Consequently, following input from the main olfactory bulb, how does the DP convey the fear response elicited by predator odor?
Combining virus tracing and patch-clamp electrophysiology, the team have discovered that DP forms excitatory synaptic connections with cholecystokinin (Cck) positive neurons in the superficial lateral parabrachial nucleus (PBNsl), which then project to tachykinin 1 (Tac1) positive neurons in the parasubthalamic nucleus (PSTh). This results in the formation of a molecularly defined tetra-synaptic pathway: MOBSlc17a7+ → DPCamk2a+ → anterior PBNslCck+ → PSThTac1+.
In order to investigate whether the tetra-synaptic pathway participate in olfaction-evoked innate fear, PhD candidates CUI Liuzhe and FENG Xiaoyang delved deeper into the functional properties of the neural circuit. Their investigations revealed that this neural circuit exhibits significant activation during TMT-induced escape behavior (Figure 3). Furthermore, optogenetic inhibition of this pathway markedly diminishes mouse escape behavior and ameliorates fear-related responses. Even in mice with concurrent damage to the cortex and medial amygdala, activation of this pathway remains capable of inducing mouse escape behavior and replicating autonomic nervous response of innate fear. These findings suggest that the identified forebrain-to-hindbrain neural circuit can autonomously regulate TMT-induced innate fear independent of amygdala.
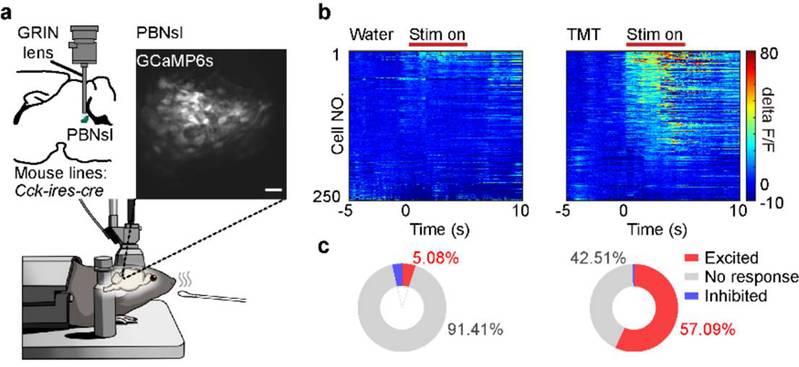
Figure 3: Single-cell calcium imaging results of anterior PBNslCck+ positive neurons when TMT is close to the mouse's nose.
As a consequence of excessive or repetitive fear contributing to fear-related disorders, such as anxiety, the research team conducted a more in-depth examination of the pathway’s function in anxiety. Dr. WANG Hao stated, “We observed that continuous optogenetic activation of this pathway (1h per day for three days) resulted in markedly observable anxiety-like behaviors in mice. Furthermore, the inhibition of this pathway led to a significant reversal of the anxiety-like behavior after 2h of acute restrain stress.”
This study revealed a tetra-synaptic neural circuit of the main olfactory bulb → dorsal peduncular cortex → lateral parabrachial nucleus → parasubthalamic nucleus, and demonstrated that this pathway can regulate olfaction-evoked innate fear and anxiety bypasses the amygdala. Prof. LI Xiao-Ming, the corresponding author of the article, believes that this research not only expands our understanding of the neural mechanisms underlying fear and anxiety but also provides new insights into the pathogenesis of mental disorders.