Recently, a groundbreaking research paper titled “Identification of an IL-1 Receptor Mutation Driving Autoinflammation Directs IL-1-Targeted Drug Design” was published in Immunity, authored through the collaborative efforts of Prof. YU Xiaomin's and Prof. ZHOU Qing’s research group from Liangzhu Laboratory of Zhejiang University.
In this study, Prof. YU Xiaomin and Prof. ZHOU Qing and their colleagues identified a new autoinflammatory disorder caused by a novel de novo missense variant p.Lys131Glu in IL-1R1. The substitution occurred on the critical positively charged amino acid, which disrupted IL-1 receptor antagonist (IL-1Ra) binding site and resulted in mutant IL-1R1 resisting to IL-1Ra inhibition. The gain-of-function mutation led to the constitutive IL-1RAP and MyD88 association with IL-1R1 upon IL-1b stimulation, and unopposed activation of the IL-1 pathway. The patient exhibited strong activation of NF-kB and MAPK pathways and overproduction of proinflammatory cytokines such as IL-1b, IL-6 and IL-8, especially in monocytes and neutrophils. Knock-in mice with Il1r1R134E/R134E presented unresponsive to IL-1Ra with upregulation of inflammatory cytokine under IL-1bstimulation and showed greater susceptibility to collagen antibody induced arthritis. They showed that dysregulated IL-1 signaling could drive pathological osteoclastogenesis, which is responsible for the pronounced defect of bone homeostasis in the development of erosive arthritis or osteomyelitis observed in the patient and Il1r1 mutant mice. Based on the diagnosis, the patient manifested with chronic recurrent multifocal osteomyelitis was treated with canakinumab (anti-IL-1b) and responded quickly with ameliorated inflammation indicated by normalized inflammatory markers CRP, ESR, and SAA, decreased proinflammatory cytokines and downregulated NF-kB signaling pathway, as well as improvement of bone lesions. Moreover, this study provided a good example of new drug design of a more specific IL-1 Trap inspired by the identified mutation in patient. Based on the mechanism of the mutation, the modification on rilonacept with the K131E mutation could generate a much more effective IL-1 inhibitor as it only traps IL-1b and IL-1a but not IL-1Ra.
Together, based on data derived from patient cells, in vitro biochemical analysis, mutant mouse models and definitive clinical responses to IL-1 targeted therapy, this study not only identified a new autoinflammatory disease caused by a novel disease causal gene IL1R1, but also clarified the disease mechanisms and provided effective treatment to the patient. In addition, this designed a new IL-1 Trap which is a more potent IL-1 inhibitor for the treatment of IL-1-driven disorders based on the mechanism of the mutation. The authors designated this disease as Loss of IL-1R1 Sensitivity to IL-1Ra (LIRSA) and the new drugas rilabnacept.
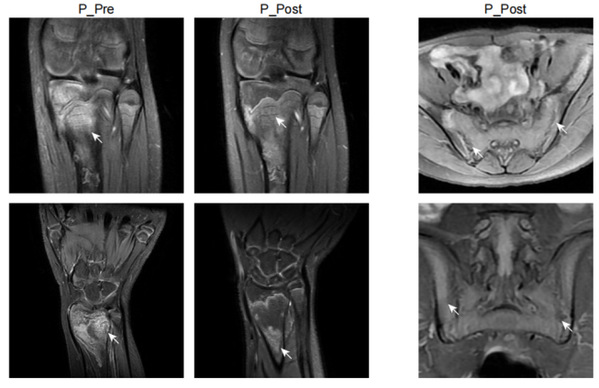
“While currently there are just a few cases, with increased awareness, more LIRSA cases will be accurately diagnosed,” stated Prof. YU Xiaomin. “Identifying the novel disease causing gene allows precise diagnosis and targeted treatment, offering hope to countless patients with rare disease, who have been seeking medical help in vain. Additionally, we hope that the new drug, rilabnacept, can swiftly enter clinical practice to provide more accurate treatment options for a broader range of autoinflammatory disease patients.”
Source: Prof. YU Xiaomin's and Prof. ZHOU Qing’s research group