Obesity is one of the global public health concerns, but many people’s weight loss plan gets stuck at the “can’t-control-their-appetite” stage. Why? Prof. WANG Hao’s team from the Zhejiang University School of Brain Science and Brain Medicine has found that a high-fat diet may cause a set of appetite-suppressing brain cells to “go on strike”, making it hard for you to resist delectable food. The study, titled “Neural adaption in midbrain GABAergic cells contributes to high-fat-diet-induced obesity”, was published on November 2 in the journal Science Advances.
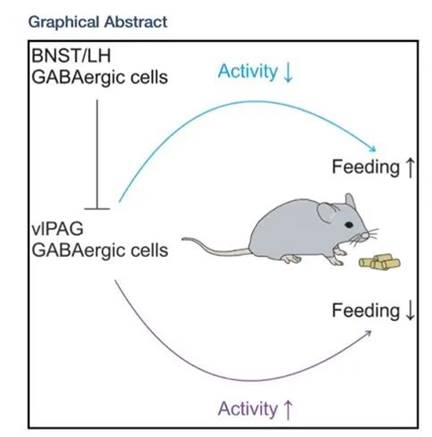
Prof. Wang’s team has long been committed to research on how the central nervous system regulates energy homeostasis. In 2019, they discovered a nucleus in the brain: the periaqueductal gray (PAG), which contains GABAergic neurons with appetite-suppressing functions. When we eat, these brain cells are also on standby, reminding us not to overeat.
The hypothalamus is an area of the brain that plays essential roles in controlling food intake. Particularly, agouti-related peptide (AgRP) neurons in the arcuate nucleus can sense hunger (energy deficits) and trigger feeding behavior. GABAergic neurons in the PAG are the downstream nuclei of the hypothalamus. When the upstream hypothalamus senses hunger, these neurons also respond and fulfill their role in supervising your food intake.
In modern society, high-calorie foods are becoming increasingly abundant, and the incidence of obesity is on the rise year by year. Diet and lifestyle changes are effective avenues of achieving weight loss, but the results are often temporary, with many people regaining weight within 5 years. “High-calorie foods may not only affect body weight and metabolism, but also result in changes in the central nervous system,” says Prof. WANG Hao. The research team explored whether high-calorie foods change our brain.
In their current study, Prof. Wang’s team discovered that with a continuous high-calorie diet, the appetite-suppressing neurons in the PAG experience a “strike”.
Their experiment was conducted on a cohort of normal-weight mice. This cohort was divided into two groups, one fed with a normal-food diet (NFD) and the other fed with a high-fat diet (HFD). After 6-8 weeks, the HFD group gained 25% more weight. Surprisingly, only after about a week of being fed with high-calorie food (no significant difference of body weight changes could be observed at this time point), the GABAergic neurons in the PAG were substantially suppressed. In other words, even before the mice’s weight changed, their brains had already undergone a change: the neurons responsible for suppressing appetites stopped working.
The “strike” phenomenon is due to an increased inhibitory synapses onto these brain cells, as well as the decreased intrinsic excitability of these cells. As a result, the mice became addicted to delicious food and couldn’t control their appetite.
Could the obesity phenotype of mice fed with HFD be reversed? Through single-cell nuclear transcriptome sequencing technology, Prof. Wang’s team found that as compare to normal chow mice, the expression of the CACNA2D1 gene in the “appetite-suppressing” neurons of obese mice was significantly reduced. When over-expressed the CACNA2D1 gene in the “appetite-suppressing” neurons in obese mice, their food intake decreased even when they still fed with high-calorie foods. Meanwhile, their weight and body fat levels also decreased. The obese mice became lean again.
The increase in CACNA2D1 gene expression not only reduced the mice’s food intake but also improved their metabolism. Therefore, CACNA2D1 has the potential to become a target for treating stubborn obesity. “If we can find molecules that can specifically and effectively activate this gene, we may well identify potential weight loss drugs.”