Comparative genomic analyses of 50 primate genomes reveal crucial genetic mechanisms of primate speciation, phenotypic adaptability, and social system evolution
●Researchers from Zhejiang University, Kunming Institute of Zoology, Northwest University, and Yunnan University, and Aarhus University have jointly led a series of significant new studies that are published in a special issue of the journal Science, and in papers in Nature Ecology & Evolution and Science Advances.
●With 27 new primate genomes assembled from long-read sequencing technology, the team has doubled the number of primate species with full genome sequences available. Comparative genomic analyses across these primate species have revealed genomic changes that have driven the evolution of the brain, social systems, and other traits.
●Comparative genomics analysis also shows pervasive conflict between the evolutionary history of individual genes and the speciation history of primates. From this incomplete lineage sorting a rich history of natural selection and the number of breeding individuals over the past 80 million years can be inferred.
●Report the first example of homoploid hybrid speciation in primates. The study strongly suggests that the Gray snub-nosed monkey was derived from hybridization event between Golden snub-nosed monkey and Black-and-white snub-nosed monkey. It highlights an underappreciated role of hybridization in new species formation and phenotypic diversity in mammals.
●Socio-ecological-genomic integrated study reveals that cold adaptation drove the regulatory changes in neurohormones signaling pathway leading to the increased social aggregation in Asian colobines. It offers new insight on the genetic basis of the social evolution in primates.
Co-led by ZHANG Guojie from Centre for Evolutionary & Organismal Biology at Zhejiang University, WU Dong-Dong at Kunming Institute of Zoology, QI Xiao-Guang at Northwest University, YU Li at Yunnan University, and Mikkel Heide Schierup at Aarhus University, the Primate Genome Consortium reported a series of publications from its first phase program which includes high quality reference genomes from 50 primate species of which 27 were sequenced for the first time. These studies provide new insights on the speciation process, genomic diversity, social evolution, sex chromosomes, and the evolution of the brain and other biological traits.

Fig.1: Phayre's langur (Trachypithecus phayrei). Credit: Guanlai Ouyang.
Large-scale phylogenomic studies reveal the genetic mechanisms underlying the evolutionary history and phenotypic innovations in primates
The comparative analysis of primate genomes within a phylogenetic context is crucial for understanding the evolution of the human genetic architecture and the inter-species genomic differences associated with primate diversification. Previous studies of primate genomes have focused mainly on primate species closely related to humans and were constrained by the lack of broader phylogenetic coverage.
“Although there are more than 500 primate species worldwide, currently, only 23 representative non-human primates species have had their genomes published, leaving 72% of genera remain unsequenced, which creates significant knowledge gaps in understanding their evolutionary history,” WU Dong-Dong states.
To address this gap, they performed high-quality genome sequencing using long-read sequencing technologies on 27 primate species, including basal lineages that had not been fully sequenced before. Combining this with previously published primate genomes, the project conducted phylogenomic studies of 50 primate species representing 38 genera and 14 families to gain new insights into their genomic and phenotypic evolution.
“Based on full genome data, we have generated a highly resolved phylogeny and estimated the emergence of crown Primates between 64.95 and 68.29 million years ago overlapping the Cretaceous/Tertiary boundary,” WU Dong-Dong states.
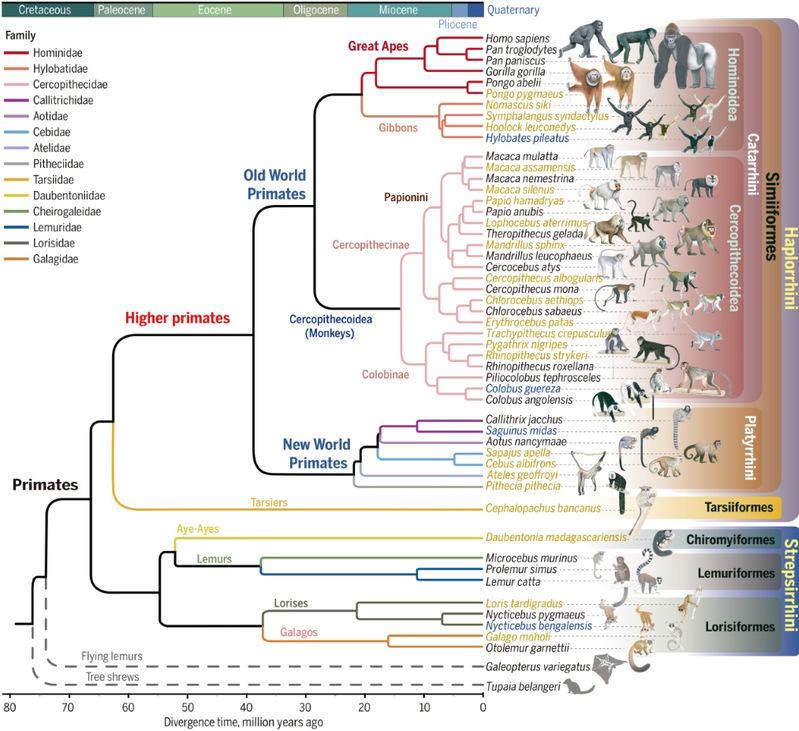
Fig.2: Genomic phylogeny of primates. Credit: WU Dong-Dong.
The study reported detailed genomic rearrangements across primate lineages and identified thousands of candidate genes that have underwent adaptive natural selection at different ancestral branches of the phylogeny. This includes genes that are important for the development of the nervous, skeletal, digestive, and sensory systems, all of which are likely to have contributed to evolutionary innovations and adaptations of primates.
“It is surprising to see that so many genomic changes involving brain-related genes occurred in the common ancestor of the Simian group which includes New-world monkey, Old-world monkey, and great apes,” states ZHANG Guojie. “These genomic innovations evolving deep in time at this ancestral node might have paved the way for the further evolution of human unique traits.”
Pervasive incomplete lineage sorting illuminates speciation and selection in primates
Although it has been well-recognized that chimpanzees and bonobos are the most closely related species to humans, 15% of our genome is closer to another great ape, the gorilla. This is primarily due to the special evolutionary event called incomplete-lineage sorting (ILS), where the ancestral genetic polymorphism randomly sorts into the descendent species. The study investigated the speciation events during the primate evolution and found ILS occurred frequently in all 29 major ancestral nodes across primates with some nodes having over 50% of genome affected by ILS.
The genetic diversification process does not follow a bifurcation tree-like topology as we normally know for speciation process, it is more like a complicated net,” ZHANG Guojie said. “It is important to investigate the evolutionary process of each individual gene, which could also affect the evolution of phenotypes across species.”
Incomplete lineage sorting (ILS) exhibits extensive variation along the genome, primarily driven by recombination. “We observed that ILS is reduced more on the X chromosome than autosomes compared to what would be expected under neutral evolution, suggesting a higher impact of natural selection on the X chromosome during primate evolution,” Mikkel Heide Schierup states.
The study exploits ILS to perform molecular dating of speciation events solely based on genome data, without fossil calibration, and found the new dating results were highly consistent with the dating with the fossil record. “This suggests that molecular dating provides an accurate estimate of speciation time even without the fossil records,” says the first author of this paper, Iker Rivas-González.
Hybridization into species events
Hybridization is increasingly recognized as an important evolutionary force forgenerating species and phenotypic diversity in plants and animals. This isespecially common in lineages that can tolerate whole genome duplication andincreased levels of ploidy. However, speciation by hybridization has been rarely reported in mammals.
Utilizing full genome data, the team discovered that the gray snub-nosed monkey Rhinopithecusbrelichi was a descendent species from the hybridization between the morphologicallydifferentiated species, the golden snub-nosed monkey R. roxellana and the common ancestor of black-white snub-nosed monkey R. bieti and the black snub-nosed monkey R. strykeri.
“To our knowledge, this is the first time that a hybrid speciation event is recorded in primates,” stated by YU Li.
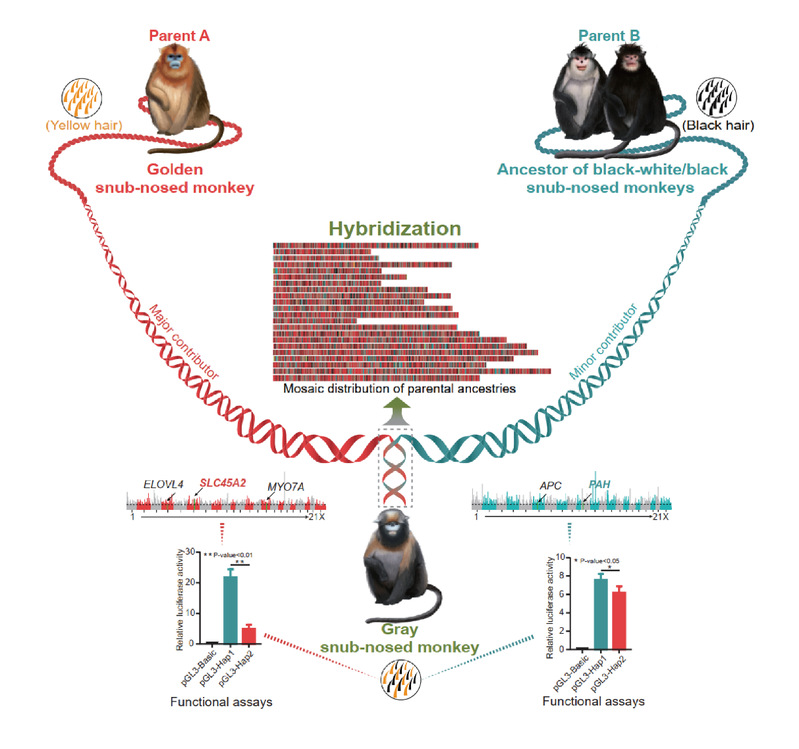
Fig.3: The hybrid origin of gray snub-nosed monkey. Credit: YU Li.
This study further identifies key genes in R. brelichi that derived from each parental lineages which may have contributed to the mosaic coat coloration in this species, and likely promoted premating reproductive isolation of the hybrid species from the parental lineages.
Multidisciplinary intersection reveals the genetic mechanisms of social complexity in Asian langurs
Primates have very diverse social systems, however the biological mechanisms underlying social evolution remain poorly known.The classical socioecological model hypothesized that the diversity of social systems evolved as a response to the environmental changes.
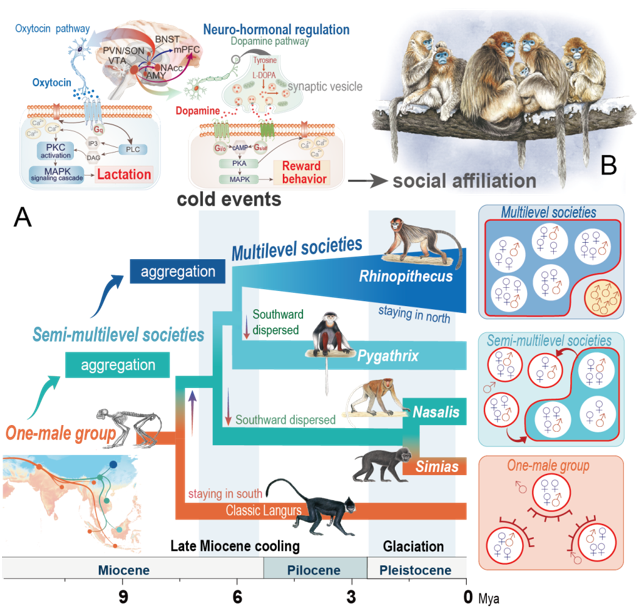
Fig.4: Cold promotes the social evolution of the Asian langurs. Credit: QI Xiao-Guang.
The study used Asian colobine monkeys as model system, as this group of species underwent a staged social evolution process from a one-male, multi-female unit to complex multi-level social forms. They have re-constructed the speciation process of this group using the full genome data and found a strong correlation between the environmental temperature and group size of the species. The primate species living in colder environments tend to live in larger groups. The ancient ice ages drove the social evolution of these primates, promoting the aggregation of spreading northern odd-nosed monkey species into nested multi-level social forms.
During this transition, odd-nosed monkeys exhibited positive selection in many genes related to cold adaptation and the nervous system. “The snub-nosed monkeys seem to have a longer mother-infant bond, which probably increased infant survival in cold environments, The DA/OXT receptors are important neurohormones in mediating social bonding. This signal pathway has been enhanced in odd-nosed monkey and promoted the social affiliation, cohesion and cooperation among adults of this species,” QI Xiao-Guang states.
Reference articles:
Shao Y, Zhou L, Li F, et al. 2023. Phylogenomic analyses provide insights into primate evolution. Science.
Rivas-González I, Rousselle M, Li F, et al. 2023. Pervasive incomplete lineage sorting illuminates speciation and selection in primates. Science.
Wu H, Wang Z, Zhang Y, et al. 2023. Hybrid origin of a primate, the gray snub-nosed monkey. Science.
Qi X-G, Wu J, Zhao L, et al. 2023. Adaptations to a cold climate promoted social evolution in Asian colobine primates. Science.
For further information and inquiries about this research, please contact:
ZHANG Guojie, Zhejiang University
WU Dong-Dong, Kunming Institute of Zoology
QI Xiao-Guang, Northwest University
YU Li, Yunnan University
Mikkel Heide Schierup, Aarhus University
Source: The research teams led by ZHANG Guojie, WU Dong-Dong, QI Xiao-Guang, YU Li, and Mikkel Heide Schierup