The crosstalk between immune cells and tumor cells within tumor microenvironment crucially determines the fate of tumor progression and metastasis. Cancer immunotherapy has brought unprecedented success in clinical benefits for multiple types of cancer by reinvigorating the functions of tumor-infiltrating lymphocytes. However, the majority of patients do not benefit from immunotherapies because of the accumulation of immunosuppressive tumor-infiltrating myeloid cells (TIMs). Emerging evidence indicates that TIMs are critical players in suppressing T cell function and promoting the tumor progression after being converted into potent immunosuppressive cells. Recently, a growing number of evidence has demonstrated that RNA N6-methyladenosine (m6A) modification plays critical roles in regulating tumor progression and tumor immunity. However, the precise regulation mode of m6A modification in controlling TIMs function is still poorly understood.
On March 22nd, Prof. WANG Qingqing, and Prof. LAI Lihua from the Institute of Immunology cooperated with Prof. DING Kefeng from the Second Affiliated Hospital, Zhejiang University School of Medicine published an article entitled Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells in the journal Molecular Cell. This study discovered that lactate in tumor microenvironment is a key factor for inducing the expression and function of RNA methyltransferase methyltransferase-like 3 (METTL3). METTL3-mediated m6A modification potently enhances the immunosuppressive functions of TIMs to promote tumor immune escape.
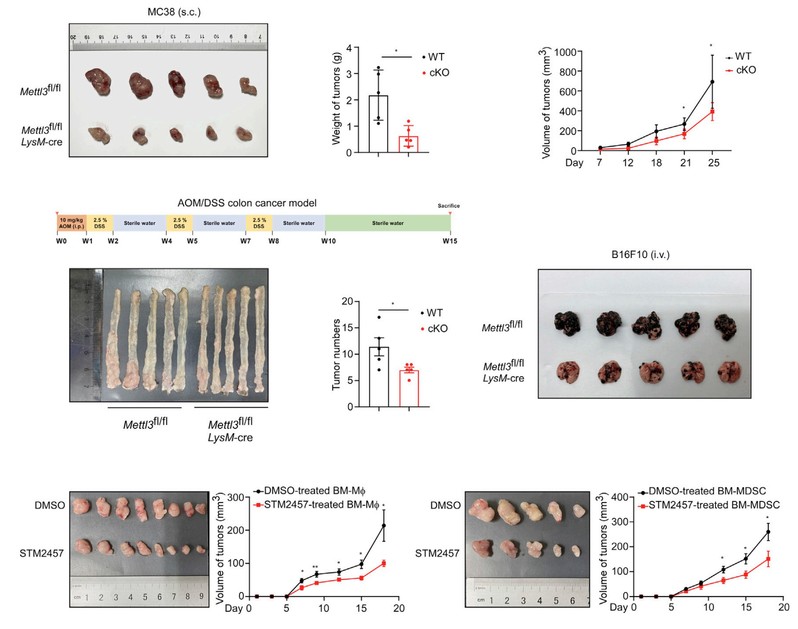
In this study, it is found that increased expression of METTL3 in TIMs was correlated with the poor prognosis of colon cancer patients and myeloid deficiency of METTL3 attenuated tumor growth in mice. METTL3-mediated m6A modification on Jak1 mRNA in TIMs, m6A-YTHDF1 axis enhanced JAK1 protein translation efficiency and subsequent phosphorylation of STAT3. Lactate accumulated in tumor microenvironment potently induced METTL3 upregulation in TIMs via H3K18 lactylation. Interestingly, the research team identified two lactylation modification sites in the zinc finger domain of METTL3, which was essential for METTL3 to capture target RNA.

Overall, the results emphasize the lactylation-METTL3-JAK1-STAT3 regulatory axis potently induces the immunosuppressive functions of TIMs. The findings in the present study led us to expect that METTL3 inhibitor might have the potential as a novel immunotherapeutic strategy for the intervention of colorectal cancer.
Source/Photo credit: the research team led by Prof. WANG Qingqing